2-Amino-4-(trifluoromethyl)pyridine
| Product name |
2-Amino-4-(trifluoromethyl)pyridine |
| Alias |
4-(TRIFLUOROMETHYL)PYRIDIN-2-AMINE;4-Trifluoromethyl-2-pyridinamine;2-AMino-4-tirfluoroMethylpyridine;-Amino-4-(trifluoromethyl)pyridine;4-Trifluoromethyl-pyridin-2-ylamine;2-AMIChemicalbookNO-4-(TRIFLUOROMETHYL)PYRIDINE;2-PyridinaMine,4-(trifluoroMethyl)-;2-Amino-4-trifluoromethylpyridineHCl;2-Amino-4-(trifluoromethyl)pyridine>2-Amino-4-(trifluoromethyl)pyridine,98% |
| CAS No. |
106447-97-6 |
| Molecular formula |
C6H5F3N2
|
| Molecular weight |
162.11 |
| Appearance |
Quasi white crystal |
| Structural formula |
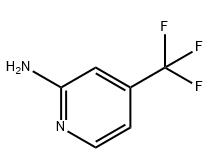 |
| Chemical property |
Melting point: 70-74 °C
Boiling point: 221.3±40.0 °C(Predicted)
Density: 1.368±0.06 g/cm3(Predicted)
Storage conditions: Keep in dark place,Sealed in dry,Room Temperature
Acidity coefficient(pKa):4.55±0.11(Predicted)
Form: Powder, Solid or Crystalline
Color: White to pale brown
BRN:7869677
|
| Security information |
Dangerous goods label: T, Xi, C
Hazard category code: 25-36/37/38-43-34-22
Safety instructions: 26-36/37-45-36/37/39
Dangerous goods transportation number: UN 2811 6.1/PG 3
WGK Germany: 3
Hazard Note: Toxic
Hazard level: IRRITANT
Hazard level: 6.1
Packaging category: II
Customs Code: 29333990
|
| Use |
2-Amino-4-trifluoromethylpyridine is an organic synthesis intermediate and pharmaceutical intermediate, which can be used in laboratory research and development processes and chemical and pharmaceutical synthesis processes.
2-Amino substituted nitrogen-containing six membered heterocyclic compounds have important applications in the chemical industry, especially 2-aminopyridine and its derivatives, which are important structures in the molecules of drugs and agricultural chemicals. They are widely used in the synthesis and application of natural products, drugs, luminescent materials, and various chemical fine chemicals. 2-Amino-4-trifluoromethylpyridine is a nitrogen containing six membered heterocyclic compound with 2-amino substitution. It is an important pharmaceutical intermediate that can be prepared by reacting 2-fluoro-4-trifluoromethylpyridine with acetamidine hydrochloride.
|

